Ricochet is the best place on the internet to discuss the issues of the day, either through commenting on posts or writing your own for our active and dynamic community in a fully moderated environment. In addition, the Ricochet Audio Network offers over 50 original podcasts with new episodes released every day.
 Learning the Basics and Things that Matter
Learning the Basics and Things that Matter
 To the sadness of all, anonymous has decided to end his amazing Saturday Night Science series (though he’s still writing for Ricochet on similar topics). Out of a sense of both depression and a desire to turn that feeling into something constructive I have decided to write a primer on something which I have found to be important in my life. I want to start out with describing a small toolbox of ideas that is taught to engineers and scientists as a means of looking at the world.
To the sadness of all, anonymous has decided to end his amazing Saturday Night Science series (though he’s still writing for Ricochet on similar topics). Out of a sense of both depression and a desire to turn that feeling into something constructive I have decided to write a primer on something which I have found to be important in my life. I want to start out with describing a small toolbox of ideas that is taught to engineers and scientists as a means of looking at the world.
Of course, there is a lot of confusion about what it is that engineers do. The truth of the matter is that there isn’t just one thing that engineers do; our professional lives are so varied and specialized that the term “Engineer” even with modifiers such as “Chemical,” “Civil,” “Mechanical,” or “Electrical” cannot capture the breadth of depth of what people do in the various disciplines. However, there are typically a few core courses which bind all of the branches of engineering together at the root of their undergraduate training. One of the most important courses which all well-trained engineers take is Thermodynamics.
Thermo gets a bad rap from the outside world. Its very name is scary, foreign, and oftentimes associated with the incomprehensible. So what is thermodynamics if it isn’t a totally baffling subject best left to boffins?
In short, Thermodynamics is the study of how energy and matter interact and the forces they consequently exert in the physical world. It is, of course, impossible to boil-down such a complex and important topic to a Ricochet post, but for those who are interested, the above link does a good job of putting flesh on the bones that I’m seeking to construct here.
There are four laws of Thermodynamics, of which only two are genuinely important for the layman. These laws explain the vast majority of the physical processes and interactions that we see in the world on a day-to-day basis (we will ignore the Zeroth and Third laws here, as they are not at the heart of my intent here).
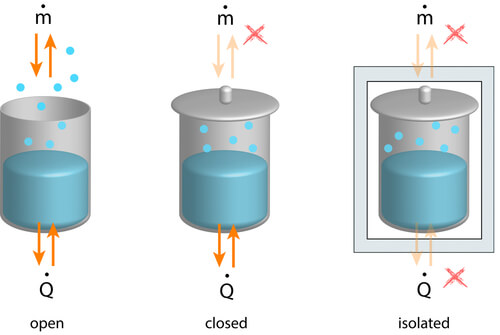
The First Law of Thermodynamics states simply that Matter and Energy are conserved. This seems fairly straightforward, and when you add in the realization (via Einstein’s famous E=mC2) that matter and energy are simply two sides of the same coin, the straightforwardness becomes even more obvious.
Why is this important? The mathematical implication of this is simple: you can use this principle to draw an imaginary boundary around a system you want assess whether or not claims being made in its name are feasible. For instance, if a traveling salesman wants to sell you a device which can power your home using nothing but the very simple and practical box in his briefcase which produces electricity out of thin air using the Kirlian effect (“Energy companies hate him… See the video before Obama bans it!”) you should not believe this man.
It’s very difficult for something to appear out of nothing, and work (a very specific form of energy) doesn’t poof into existence any more than gold or water do.
In case the First Law is old hat to you, there’s always the Second Law of Thermodynamics. There are several ways to describe it but — in its simplest form — it states that a cold cup of coffee left on a kitchen counter will not spontaneously heat-up. Another way of saying it is that you cannot build a machine whose sole effects are the cooling of a heat sink and the lifting of a weight.
Huh? Well, in the first case, this ought to match with your day-to-day experience. Let’s say you’ve made coffee upon waking up and then had to help you kids get ready for school, forgetting your coffee on the counter. While the coffee can — and will — cool down if left to its own devices, it cannot — and will not — heat back up to its previous temperature barring some outside intervention.
Why is that? Energy, much like water “seeks its own level,” and it tends to flow from points of high energy into points of lower energy. Picture, if you would, a clear hose which you fill partway with water while holding part of it out of the water. Now put your thumb over the submerged end and hold the two ends of the hose level with one another. Then let your thumb off of the previously submerged side – like so:
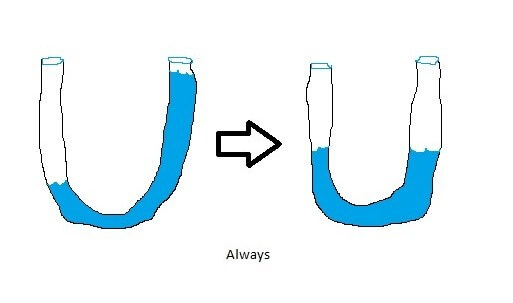
The water always seeks its lowest energy state: it does not spontaneously pile up in one side of the hose. This is the same phenomenon that is on display with your coffee cup.
The other way of describing the Second Law that I mentioned discusses heat and its relation to work. “You cannot construct a machine whose sole effects are the cooling of a heat sink and the lifting of a weight” means simply that you cannot completely convert heat energy into work. That would imply a completely adiabatic (and isentropic) process.
The implication of this expression of the Second Law is that energy has a Quantity (covered by the First Law) and a Quality (the realm of the Second law.) The Quality of energy has to do with how much work which you can extract from it. Entropy (the general implication of the Second Law) is a measure of the energy in a system which can’t be converted into useful work.
So how can this information be put to use in your life? Much like internet ads that promise you limitless energy for your home for free, occasionally you’ll hear stories about somebody who has finally come up with a perpetual motion machine – a machine that will continuously function without energy input in the same fashion forever. Think of a fan blade inside of an evacuated glass bell jar which spins and spins eternally:
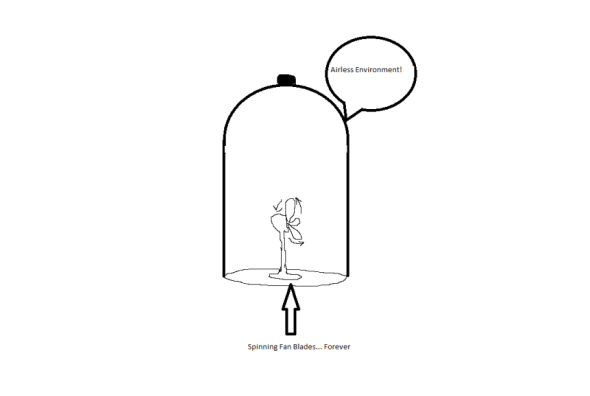
Obviously, this is fiction, as you would need a frictionless connection between the fan blade and the machine. Nonetheless, such things are still sought after.
You might be wondering, how does this inform my worldview? Well, very simply, using these concepts — something doesn’t come from nothing; order in a closed system eventually becomes disorder; etc. — you can begin to assess the truthfulness of some of the wilder claims which you might be confronted with.
From where did the energy powering a device or process come? How is it affecting the environment? Do your suppositions about that violate one of the laws? Keeping those things in mind will banish a significant amount of balderdash.
Published in Religion & Philosophy, Science & Technology



Never heard of them. Finally watched the video. Fantastic and funny. Will have to check them out.
http://www.amazon.com/Complete-Flanders-Swann/dp/B006CII29U/
And yet, despite this simple explanation, people still think that Big Oil is still hiding the patent on the car that runs on water.
-E
I don’t think they can’t be helped unfortunately. These are frequently the same people who buy organic produce and wonder why they’re broke.