Ricochet is the best place on the internet to discuss the issues of the day, either through commenting on posts or writing your own for our active and dynamic community in a fully moderated environment. In addition, the Ricochet Audio Network offers over 50 original podcasts with new episodes released every day.
 Learning the Basics and Things that Matter
Learning the Basics and Things that Matter
 To the sadness of all, anonymous has decided to end his amazing Saturday Night Science series (though he’s still writing for Ricochet on similar topics). Out of a sense of both depression and a desire to turn that feeling into something constructive I have decided to write a primer on something which I have found to be important in my life. I want to start out with describing a small toolbox of ideas that is taught to engineers and scientists as a means of looking at the world.
To the sadness of all, anonymous has decided to end his amazing Saturday Night Science series (though he’s still writing for Ricochet on similar topics). Out of a sense of both depression and a desire to turn that feeling into something constructive I have decided to write a primer on something which I have found to be important in my life. I want to start out with describing a small toolbox of ideas that is taught to engineers and scientists as a means of looking at the world.
Of course, there is a lot of confusion about what it is that engineers do. The truth of the matter is that there isn’t just one thing that engineers do; our professional lives are so varied and specialized that the term “Engineer” even with modifiers such as “Chemical,” “Civil,” “Mechanical,” or “Electrical” cannot capture the breadth of depth of what people do in the various disciplines. However, there are typically a few core courses which bind all of the branches of engineering together at the root of their undergraduate training. One of the most important courses which all well-trained engineers take is Thermodynamics.
Thermo gets a bad rap from the outside world. Its very name is scary, foreign, and oftentimes associated with the incomprehensible. So what is thermodynamics if it isn’t a totally baffling subject best left to boffins?
In short, Thermodynamics is the study of how energy and matter interact and the forces they consequently exert in the physical world. It is, of course, impossible to boil-down such a complex and important topic to a Ricochet post, but for those who are interested, the above link does a good job of putting flesh on the bones that I’m seeking to construct here.
There are four laws of Thermodynamics, of which only two are genuinely important for the layman. These laws explain the vast majority of the physical processes and interactions that we see in the world on a day-to-day basis (we will ignore the Zeroth and Third laws here, as they are not at the heart of my intent here).
The First Law of Thermodynamics states simply that Matter and Energy are conserved. This seems fairly straightforward, and when you add in the realization (via Einstein’s famous E=mC2) that matter and energy are simply two sides of the same coin, the straightforwardness becomes even more obvious.
Why is this important? The mathematical implication of this is simple: you can use this principle to draw an imaginary boundary around a system you want assess whether or not claims being made in its name are feasible. For instance, if a traveling salesman wants to sell you a device which can power your home using nothing but the very simple and practical box in his briefcase which produces electricity out of thin air using the Kirlian effect (“Energy companies hate him… See the video before Obama bans it!”) you should not believe this man.
It’s very difficult for something to appear out of nothing, and work (a very specific form of energy) doesn’t poof into existence any more than gold or water do.
In case the First Law is old hat to you, there’s always the Second Law of Thermodynamics. There are several ways to describe it but — in its simplest form — it states that a cold cup of coffee left on a kitchen counter will not spontaneously heat-up. Another way of saying it is that you cannot build a machine whose sole effects are the cooling of a heat sink and the lifting of a weight.
Huh? Well, in the first case, this ought to match with your day-to-day experience. Let’s say you’ve made coffee upon waking up and then had to help you kids get ready for school, forgetting your coffee on the counter. While the coffee can — and will — cool down if left to its own devices, it cannot — and will not — heat back up to its previous temperature barring some outside intervention.
Why is that? Energy, much like water “seeks its own level,” and it tends to flow from points of high energy into points of lower energy. Picture, if you would, a clear hose which you fill partway with water while holding part of it out of the water. Now put your thumb over the submerged end and hold the two ends of the hose level with one another. Then let your thumb off of the previously submerged side – like so:
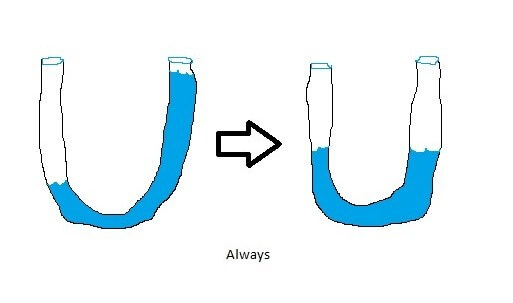
The water always seeks its lowest energy state: it does not spontaneously pile up in one side of the hose. This is the same phenomenon that is on display with your coffee cup.
The other way of describing the Second Law that I mentioned discusses heat and its relation to work. “You cannot construct a machine whose sole effects are the cooling of a heat sink and the lifting of a weight” means simply that you cannot completely convert heat energy into work. That would imply a completely adiabatic (and isentropic) process.
The implication of this expression of the Second Law is that energy has a Quantity (covered by the First Law) and a Quality (the realm of the Second law.) The Quality of energy has to do with how much work which you can extract from it. Entropy (the general implication of the Second Law) is a measure of the energy in a system which can’t be converted into useful work.
So how can this information be put to use in your life? Much like internet ads that promise you limitless energy for your home for free, occasionally you’ll hear stories about somebody who has finally come up with a perpetual motion machine – a machine that will continuously function without energy input in the same fashion forever. Think of a fan blade inside of an evacuated glass bell jar which spins and spins eternally:
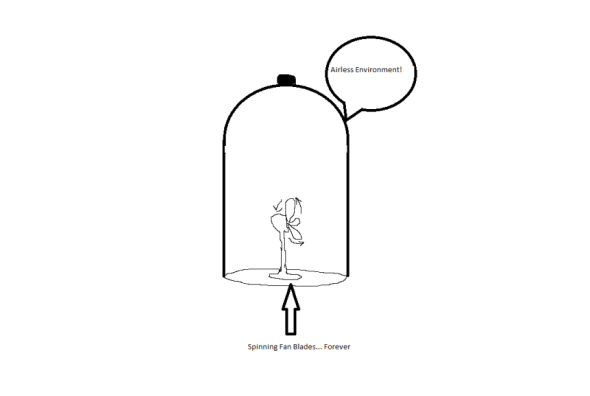
Obviously, this is fiction, as you would need a frictionless connection between the fan blade and the machine. Nonetheless, such things are still sought after.
You might be wondering, how does this inform my worldview? Well, very simply, using these concepts — something doesn’t come from nothing; order in a closed system eventually becomes disorder; etc. — you can begin to assess the truthfulness of some of the wilder claims which you might be confronted with.
From where did the energy powering a device or process come? How is it affecting the environment? Do your suppositions about that violate one of the laws? Keeping those things in mind will banish a significant amount of balderdash.
Published in Religion & Philosophy, Science & Technology



“There ain’t no such thing as a free lunch.”
Under that heading, can we do away with the notion of the “Keynesian Multiplier” under the heading of the First Law of Thermodynamics? A Scientific Law destroying a macroeconomic fallacy?
Maj:
This is restraint of trade. With this sort of propaganda I’ll never get rich off of my heatcooldisorandorderedconservingwastingperpetualthingambobby.
My spelling proves disorder increases over time.
Does not Keynesian economic policy eventually embrace entropy?
Don’t answer that.
In the long run we’re all dead.
Statistical mechanics?
Sure, if you want to get down to the most elementary definition, Thermo is defined by the interaction of particles on an atomic level. A perfect example of this phenomenon being that “average temperature” as we conceive of it is the statistical average energy of a bunch of particles with very different energies. Worrying about that is a fine detail however, and masks the fact that we statistically infer that a body of matter has the statistical property we can measure called “temperature” because we have no practical means of separating out the more energetic (higher energy particles) from the slower, less energetic particles and thus gain the ability to have them perform work.
For all intents and purposes, the average temperature of matter is functionally similar enough to matter whose particles all have identical kinetic energy to be considered the same.
An example of how they might be different is in the case of, say, water molecules superheated in a microwave to an average temperature below that of water’s boiling point, but which spontaneously can erupt if perturbed because in addition to the kinetic energy of the particles themselves, microwaves have the effect of spinning the molecules, and that spinning energy can be very quickly translated into an increase in temperature in certain conditions.
For me, intro to stat mech was more intuitive than intro to therm. Maybe because I liked seeing the statistical inference out in the open?
But it’s good to see you write on science. I’m wondering whether several of us shouldn’t step up and fill the void – even asked Tom about it. We need a geek squad ’round here.
Two thoughts upon reading your post:
1. I’m saving all of these in a folder for my 14 year old future engineer son to read this summer.
2. Leaving Ga Tech was SO the right move for me.
Ok, one more…
Thanks, this is really interesting and (mostly) understandable. The mostly is my deficit, not yours.
I’m sure there’s a term for this phenomenon, but it’s what occurs to people who are steeped in a particular worldview or set of assumptions. They project similar assumptions upon other people under the false pretense that their experiences and everybody else’s are comparable. “Epistemic Closure” Perhaps?
I have to stop myself frequently because it is not accurate to think that most people have had even high school physics, let alone calculus and thermo. All of these are powerful tools that will allow you to see through flimflammery.
Is there a link somewhere to this announcement? I missed it.
LW calls it the typical mind fallacy (yes, religious people can appreciate LW, too – though to be honest I know of few that do). “Epistemic Closure” gets used in politics thusly, and that’s more about making your priors update-proof, I think. (Not at my brightest lately, so could be wrong.)
Is there anything that I can do to clear something up for you?
If you read carefully John’s last entry on the Russian Rocket Program you can find it buried there, quite skillfully.
Embrace the cycle.
Phase change is where the magic happens. Without it the discipline wouldn’t be so awesome.
The most interesting thing I learned in the Navy was how the Carnot cycle worked, and how much its basic premises applied to most walks of life. It taught me that extracting anything out of anything is hard, you don’t get nearly as much as you give, but that this is simply the way things are. That curvy parallelogram haunts me to this day.
There might not be perpetual motion, but you can cleverly position yourself near the phase changes to create a simulacrum.
Embrace limitations, turn them into strengths. There is nothing new, only clever arrangements of the timeless.
It’s the “feature, not bug” view on things.
Doesn’t it cause a fallacy if we try to to apply laws of Thermodynamics outside of physics?
Are you trying to tell me there is no such thing as a Sky Hook?
Yeah, women hear that one a lot.
To top that off, most people in this country have never in their lives done any physical work. So they have not observed cause and effect in that context.
Thank you for posting this.
I was enjoying this article until I got to the hose drawing. Then I began to enjoy it immensely. Thanks.
Maj sure has a way with a hose :-) They were adorable.
Not at the moment, it’s secondary to allergies which make me stupid : )
See, I’m hearing this as “Maj sure has a way with hose”. Frankly, I don’t want to be.
I got a “C” in my college Thermo class. Of all the stupid mistakes I made in college I regret that one the most bitterly.
There’s a famous question of thermodynamics: If you’ve got a refrigerator in an insulated room and you leave the door open, does it get warmer or colder?
My father asked us kids the question when I was ten. I heard it again in high school physics, in college physics, at the tech school, and was asked it twice in job interviews (at the same company.)
The answer, for those of you playing at home, is that it gets warmer. Any coldness the fridge exudes out the front is more than covered by the heat it’s dumping out the back. To make the fridge colder you have to make the room hotter, and you always lose in that game.
Well, it obviously gets warmer. The question could be interesting on an exam on the basis of what control surface you choose to frame the question with. Also, tell the students that the door is shut. ;)
This also reminds me of the semester that I took Thermo – it was really hot that September and August (1998) and the dorms at Mines didn’t have air conditioning, so some enterprising students took their cube refrigerators and stuck the coils out the window while leaving the door to the fridge open. Add in a fan, and you have a weak air conditioner.
If you think about it correctly then there is no fallacy. First, money is merely a physical manifestation of human labor. It represents a promise to do a certain amount of labor in return for that paper (the paper’s implication being that it is a stand-in for intrinsic value such as bushels of wheat or bars of gold.)
The Keynesian argument is this: Essentially, that turning loose a flood of money on the economy will actually create MORE economic activity than the initial input of money – thus creating work out of nothing. The very term “Keynesian Multiplier” implies that if you spend $1.00 in the economy that this dollar has ripple effects which are amplified throughout the economy such that by the time you perform the final accounting, the initial dollar spent actually ends up as $1.06 of economic activity.
This is a sort of legerdemain which only works so long as you pretend that the value of money is constant and doesn’t inflate. However, Keynesians (being clever) still advertise that their after-inflation ROR on a dollar spent is somehow positive.
This is a miracle – as surely as striking a rock and having a river gush forth from it is miraculous. I think this makes Keynesians “Science Deniers.”
Oh, thanks. I didn’t read that, as I’m not all that interested in Russian rockets…
There is no “outside of physics”.
Heaven and Hell, perhaps…
Maj,
In 1899 Max Planck had a mess. It was where Physics was left after the behavior of waves (or particles) began to be known. He started fresh with his Quantum idea. He also thought he could hang onto something from Classical Physics. It was the 1st and 2nd laws of thermodynamics. He hung onto them.
Thought you might like to know.
Regards,
Jim