Ricochet is the best place on the internet to discuss the issues of the day, either through commenting on posts or writing your own for our active and dynamic community in a fully moderated environment. In addition, the Ricochet Audio Network offers over 50 original podcasts with new episodes released every day.
 The Future Is (Almost) Here
The Future Is (Almost) Here
 Since the first discovery that our physical traits are determined by a four-letter DNA code, and since our first attempts to manipulate that code, the idea of genetically-designed humans has been a staple in the science fiction realm. But among biomedical researchers, the notion of engineering DNA in actual humans remained strictly hypothetical: while all agreed it was theoretically possible, the methods available were too cumbersome, inefficient, and limited to too few species (such as mice) to imagine a realistic path forward.
Since the first discovery that our physical traits are determined by a four-letter DNA code, and since our first attempts to manipulate that code, the idea of genetically-designed humans has been a staple in the science fiction realm. But among biomedical researchers, the notion of engineering DNA in actual humans remained strictly hypothetical: while all agreed it was theoretically possible, the methods available were too cumbersome, inefficient, and limited to too few species (such as mice) to imagine a realistic path forward.
Until now.
A revolution in the nascent field of nuclease-based genome editing is currently taking place, and even the most skeptical researchers are now proclaiming that we will be regularly manipulating DNA in adults within a few years, and that the first genetically-engineered babies may be a reality within the decade. And unlike previous claims of world-altering advances which promised we would soon be flying in autonomously-guided solar-powered cars while receiving a massage from our personal robot, nuclease-based genome editing has already passed several previously-insurmountable hurdles: genetically-altered monkeys were generated last year, demonstrating feasibility in our closest biological relative. And astonishingly, just three weeks ago a group in China reported successful genome editing in human embryos.

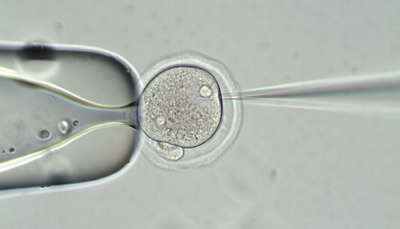
Liu et al. (2014), Cell
This rapid progress owes its success to an ingenious new paradigm. As so often in molecular biology, the most elegant and efficient way to carry out a nearly impossible task is to find a way for nature to do it for you. These new methods take that truism one step further by combining two completely unrelated natural phenomena: a bacterial “immune system,” and the ability of cells to “heal” their DNA when it is injured.
Instead of the antibodies, T-cells, and macrophages used as weapons by our human immune systems, many bacteria have a much more rudimentary defense: identify foreign invading DNA and cut it up using enzymes known as nucleases. Meanwhile, in a complementary but biologically unrelated phenomenon, the cells of all higher organisms have intrinsic methods to repair cut DNA. And like a cut on the skin, when cut DNA heals, it leaves a scar – except that the genetic code is so sensitive that this scar usually inactivates the gene being healed. Moreover, just like a skin wound, it is possible to artificially “graft” a new sequence of DNA into the wound, thereby allowing a new gene to be inserted into the genome.
Thus, a genome is edited by artificially introducing the components of this bacterial immune system into human/primate/mouse/etc. cells and targeting them to the gene of interest – and then letting the nature do the rest. This is the general principle underlying several different methods which have made news since 2010, including ZFNs, TALENs, and CRISPR/Cas9. While each of these methods is slightly different, they are all orders of magnitude simpler, cheaper, safer, more efficient, and more versatile than previous techniques. CRISPR, the newest and most promising of the methods, allows any site in the genome to be targeted, and only requires several days of preparation using simple molecular biological methods. Its basic framework also allows it to be delivered to nearly any type of cell from nearly any species.
There are still several kinks in the methods. Currently, only cells in a Petri dish can be manipulated, not in the organism itself. Off-target mutations may also occur, and the extent and mechanism of this problem remain unclear (off-target mutations were an issue in the experiments on human embryos). However, given the incredibly fast speed at which these methods have been developed, it is reasonable to expect that these issues will also be cleared up in time.
But regardless of any technical road bumps ahead, the success with genetically-manipulated monkeys and human embryos clearly demonstrate that the impossible is now possible: we now have a technology that we can use to change our own DNA.
This brings us to the ethical questions of genome editing. The implications of being able to rewrite any section of DNA and to produce “designer babies” are obvious even without an understanding of the underlying science. In a promising development, all of the leading US and European researchers have agreed to a moratorium on experiments in human embryos. Even the Chinese group at least minimized the ethical ramifications of their human experiments by only using leftover embryos from IVF which had been fertilized by 2 sperm each – meaning they were incapable of sustaining human life even before the experiment.
Here in the US, human research is focusing on genome editing in (non-embryonic) induced pluripotent stem cells. In this situation, cells (usually skin or blood) are harvested from a patient with a genetic disease, converted to stem cells, the DNA defect is corrected in these cells Petri dish, and then the cells are induced to differentiate to the specific type of cell affected by the disease (say, a neuron or a lung fibroblast) and re-introduced into the body. This is an ethically impeccable approach and will likely work well for certain cell types and diseases (such as leukemias), and phase II have already begun to test genome editing as an approach to counter HIV infection. However, for other diseases the last two steps of the process – converting the stem cells into specific cell types and placing them in the correct context back into the body – remains incredibly difficult. For that reason, altering the DNA in the embryonic stage (or of the sperm and egg which create it) will always remain an incredibly tempting approach.
And even many of the scientists currently calling for a moratorium believe that manipulation of human embryos (and thus permanently changing our genetic lineage) is inevitable. Indeed, given human nature, it is naive to think that such advances can be stopped – and the incredible efficiency of these methods (which thereby require fewer embryos to be destroyed) will only lower any ethical thresholds. Given the breathtaking pace of these technologies, it is incumbent on us to discuss their ramifications now, and not later.
What do you think? Should we impose any restrictions on genome editing? Should the medical profession try to voluntary restrict itself to only performing certain genetic interventions?
Published in General



This concern is already being very actively addressed, and is likely very manageable.
Since we now have several thousand full human genome sequences, we not only “know” the entire sequence but also have a good idea of inter-individual variation.
As a result, there are already a number of online tools available to develop the target sequences used for CRISPR and the other nuclease-based technologies which specifically ensure that the target sequence which is to be mutated (or a close analog of that sequence) is not found elsewhere in the genome. Furthermore, cytogenetics techniques could be used on each patient prior to treatment to confirm that the target sequence is not found anywhere else in their genome except in the desired location.
A bigger concern currently is infidelity of the nuclease systems themselves. It is still unclear how often, where and why these systems might introduce mutations into sites in the genome which would not be predicted target sites. That is currently one of the main foci of new research going forward.
I almost signed up for one, since I carry a mutation for HIV resistance that might help some lucky HIV+ cancer patient (if “lucky” describes someone who’s HIV+ with cancer in the first place). But as far as I can determine, I fail to meet donor requirements.
Supposing donor requirements change (or there’s a laxer registry out there that I don’t know about), I don’t mind the thought of my marrow being used to “edit” another person’s immune system – though I’d want to be sure that editing an immune system to make it more like mine was really an improvement!
Regarding unintended consequences – with the techniques described, is it certain that no other changes occur anywhere else? For example, can a similar sequence of codons, elsewhere (even on another chromosome) be changed as well? It seems to me (and I could be wrong) that an enzyme which splice genes is not specific to every single gene and must therefore attach to given sequences of base pairs, which occur in many loci. If so, the effect of other changes in base pairs may not be apparent, unless immediately expressed phenotypically as, say, if a recessive trait arises or if the change occurs in a regulatory segment. If this is possible, I would imagine that germ cell therapies could result in even more unforeseen changes in future generations. So, does the current state of the art rule this out?
Are you homozygous for the delCCR5 mutation? That would be cool.
However, I think you’re conflating two approaches. The type of trial which would recruit you is looking for donors with the “HIV resistance” mutation to donate stem cells, which would then be transplanted into HIV patients after destroying their immune system by irradiation. However, this approach still poses the problems of a) finding suitable donors, and b) host-donor incompatibilities.
With genome editing, there’s no longer any need for a donor – the stem cells would be taken directly from the HIV patient, the delCCR5 mutation would be engineered into those cells, and those cells would then be re-transplanted. In other words, the HIV patient is his own donor, which eliminates the majority of the problems surrounding transplantations.
Yes.
No, I realize the approaches are different. It’s just that the ethics involved strike me as similar. I realize the advantages genome editing has over transplantation, but it seems to me that, in both cases, we are genetically editing the one being treated – for isn’t a successful recipient of donor organs now a kind of genetic chimera?
Sorry if my previous comment was unclear.
Systems like CRISPR/Cas9 and ZFNs are targeted to specific nucleotide sequences – in the case of CRISPR, the sequence is 23 nt long. There is no inherent “gene specificity”, only specificity for that 23-nt sequence – thus, if it occurs multiple times in the genome, each of those sites will become mutated.
However, since we know the sequence of the entire human genome, this problem is theoretically easily solved: just look for a unique sequence within the gene of interest, as almost every gene does indeed contain signatures which are unique throughout the genome.
How well this pans out in practice is currently under investigation. Even if the target sequence is unique, if the CRISPR system also occassionally targets sequences with 1 nt difference, that throws a big wrench into the system.
Furthermore, there is the thorny issue of having two alleles for each gene: if the allele on one chromosome is diseased but healthy on the other, is it possible to only target the disease-causing allele (assuming that the disease is caused by a point mutation, and that the sequences of the two genes are otherwise identical)?
Sorry, I was thrown off by your using the term “editing” in the context of transplantation. Because editing is now being used as a technical term to specifically mean manipulation of nucleotide sequences within a genome, I would not use it with respect to transplantation. But your general point is correct: both methods would result in transgenic (and chimeric if only certain cells are edited) individuals.
As to the philosophical issue, I’m not sure they’re exactly identical. Directly changing our own DNA sequences feels like “playing God” in a way – we can alter our DNA to create a genome which never has and otherwise never could exist. In contrast, transplantation is still limited in selection to the genomes currently in existence within the population.
And technically correct is the best kind of correct…
No, of course I understand how my colloquial abuse of your term of art would cause you to scratch your head :-)
Not exactly identical, I agree.
But I see important similarities, and I also see motivation for conservatives to conveniently ignore those important similarities in order to advance a spuriously “ironclad” argument against one while also defending the other as wholesome, pro-life established medicine.
Score!
Hey, men, isn’t it nice to know that science agrees that all women are freaks? ;-)
At the risk of sounding like a politically-incorrect oaf for judging you by your genetic background, may I just say: I’m in awe.
Virologists talk all the time about people homozygous for the deletion in CCR5 – how much research has been done on them, what future research would be possible, etc. Yet I have never met a researcher who actually had contact with a subject with that genetic makeup. It’s like the virological Yeti.
In all seriousness, even if you didn’t enroll in that particular clinical trial, I am certain that there are a number of HIV researchers who might be interested in contacting you. It’s difficult to identify specific groups currently interested, but I imagine if you are near a major research hospital with an HIV team that they could point you in the right direction.
Can you explain what this even is, why it is so rare, and why it is worth looking for?
These developments are exciting and scary at the same time! There is so much potential for good, but I confess to having some fear about the potential for harms.
Thanks for posting, Mendel.
Prayers for your Mama, MJ, and thanksgiving for the advance.
Viruses gain access to the interior of their target cells by docking onto specific surface proteins on the outside of those cells.
In the case of HIV, there is a protein on the surface of many immune system cells called CCR5 (C-C chemokine receptor 5) which the virus latches onto in order to infect its target cells (mostly T cells).
Some people have a genetic mutation in which a small part of this protein is naturally missing. The deletion renders the protein non-functional (although its carriers remain healthy), thereby preventing the virus from gaining access to the cell, thus rendering these subjects effectively resistant to HIV infection.
But there’s a wrinkle: the mutation is somewhat rare as it stands. But we all carry 2 copies of each gene, and in order for an individual to be resistant to HIV, both copies of the gene have to carry this deletion (meaning both the father and the mother had to have at least one copy of the mutation). The chances that both copies of the CCR5 gene being the mutated version in one individual are thus quite low – I believe the prevalence is less than 1% of the population.
(con’t from above)
A few years ago, there was a remarkable case in Germany in which an HIV-positive leukemia patient had his entire immune system irradiated and replaced by bone marrow from a donor with the CCR5 deletion mutation. That patient was then taken off antiretroviral drugs, and has now survived almost 5 years virus-free with no medication.
Based on that success, several clinical trials have been recruiting donors like Midge to try and replicate the results. However, since the procedure requires both temporarily knocking out the HIV patient’s immune system and performing bone marrow transplantation, it is both very risky and very expensive.
As a response, Sangamo, a startup company here in the Bay Area, is using new gene editing technology (specifically ZFNs) to remove HIV patients’ T cells from their blood, artificially engineer the CCR5 mutation into those cells in the lab, and then return the same cells back to the patients. The hope is that they will then have enough HIV-resistant T cells to allow them to go off their medications without needing a bone marrow transplant. Preliminary results were promising, but this is still all very nascent.
Great conversation, Mendel. Some of the practical, legal and cultural aspects of genetic engineering are described in Peter Huber’s excellent book, The Cure in the Code, How Twentieth Century Law is Undermining Twentyfirst Century Medicine. He also had a great article on stem cell research you can find at the City Journal website.
It is advances like these that make me have hope for my son’s schizophrenia being treatable in his lifetime. For those that argue we don’t need more H-1b visas for scientists that could move the ball forward in this field [and provide support staff, lab tech and other employment for several more Americans in the process] I wish they would look at the issue from the perspective of my family.
It seems to me this conversation has only traveled in one direction, but has missed what was, to me, the important aspect of Mendel’s post, for general discussion.
As a kid, I consumed everything Isaac Asimov wrote and have become dismayed at the extent to which the world has given attention to his minor plot devices and missed the important things I thought he was trying to say. That guy was being published by the age of seventeen, long before he became a professor of biochemistry. He spent a great deal of mental energy on the concepts of ethics and morality, in science. Our world is failing him, when it comes to aspects of robotics and artificial intelligence.
Our societies have failed to create mores and a system of ethics and we will rue this failure, I believe.
Mendel asked simple questions:
“What do you think? Should we impose any restrictions on genome editing? Should the medical profession try to voluntary restrict itself to only performing certain genetic interventions?”
These are the important questions. I first did my own recombinant experiments on strands of DNA in 1985. Other people did them long before me. Just because we can do something does not make it right. As our world rushes past Asimov’s cautions with respect to robotics and artificial intelligence, having yet to establish a protocol, we now are entering the phase where we are toying with his core subject, biochemistry. With no mores or ethics having been established.
I just hope we apply the same reasoning to government regulation.
Really? I see many protocols being established in technical fields through the ordinary process: evolving custom.
Or is a protocol only a protocol when it’s imposed?
“Really? I see many protocols being established in technical fields through the ordinary process: evolving custom.”
You are more of an optimist than I, but I think you are on to something there. Instead of feeling frustrated by Huber’s description of the stifling effect of the regulatory bureaucracy on genetic development, I am amazed at how vibrant the field is despite that burden. If I weren’t a pessimist I would think that the ethical dilemmas might resolve themselves through evolving custom. I pray you are right.
Not to go full Luddite here, but isn’t this the opening sequence to a lot of movies and dystopian stories? What is to restrain this technology from being used for evil?
Truly in awe of the depth of conversation about this topic. I use these systems regularly as a research tool, but would be hard pressed to have such an organic conversation with my colleagues. I’ve also never been able to discuss the philosophical ramifications of their use as treatment, so here goes: how much editing can we do without altering our Humanity? How big of a role do disease and mortality play in making us who we are as individuals, groups, even nations?
I’m still waiting for cold fusion or any kind of useful fusion. I am with Chris Johnson#50 we are currently without an ethical or legal frame work to deal with these new advances. The reality is these and other genetic advances will occur and ethical and legal standards will be created after the fact. I can see the commercials now, “were you or a loved one genetically modified? Are your vital organs slowly ceasing to function? You or your family maybe entitled to significant compensation. Call 1 800 aa-aagh. Operators are standing by.”
Our DNA is a 4 letter word. Anyone else find that funny?
I wouldn’t be surprised to see a history of new technology -> dystopian future predicted by fiction -> technology turns out to be unambiguously awesome.
Not “successful”:
Not to pick—too much—on Mendel, but this reminds me of the early claims for cancer research. CTD
Cancer research has largely been a failure. The early claims assumed that we knew what caused cancer. We’ve since learned that those early assumptions were in error, and we still know little about what causes most cancers—there have been a few successes.
The safe bet is that the same will hold true for manipulation of the genome. There are indeed a few fixes that prove to be “easy”, as above, but most will turn out to be based on erroneous assumptions. At a point in the science when there’s a fight over if 98% of the genome is even functional—I’m guessing it is—it’s a little early for successful manipulation.
Imagine re-writing computer code when you don’t understand the entire language used in the computer. How is that likely to turn out?
So this post has a bit of the tone of Progressive revival-tentism: Yay, look what wonderful things will come from science!
But indeed, let research continue—and keep the regulators away. It’s the only way to find those few needles.